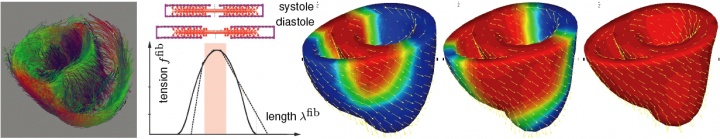
cardiovascular disease is the primary cause of death in the industrialized world, generating annual health care costs in excess of $430 billion in the united states alone. in spite of tremendous scientific improvements during the past 20 years, heart failure remains one of the most common, costly, disabling, and deadly medical conditions affecting more than 25 million people worldwide. repairing the diseased heart is a desirable but elusive goal. traditionally, the development of cardiac therapies has been experience-based empirical rather than simulation-based predictive. advanced continuum theories combined with modern imaging modalities and computational techniques now offer the potential to provide greater insight into the complex pathways of cardiac disease, and thereby guide the design of new successful treatment strategies.
our vision is to establish computer-guided surgical planning in cardiac disease using novel multi-scale continuum theories of excitation, contraction, and remodeling.
in the united states alone, almost half a million people die each year as a result of cardiac arrhythmias.
in the healthy heart, rhythmic cardiac contraction is coordinated
through smoothly propagating electrical waves of excitation.
to model the spatio-temporal evolution of cardiac excitation, we adopt the modified fitzhugh-nagumo equations for excitable cells.
they define a system of coupled evolution equations for the fast action potential
and the slow recovery variable which phenomenologically summarizes the effects of all ionic currents across the cell membrane.
we have developed a novel finite-element based solution technique in which the action potential is introduced globally as a nodal degree of freedom, whereas the recovery variable is treated locally as an internal variable on the integration point level. paired with an implicit time integration scheme and an incremental iterative newton-raphson solution strategy, this particular discretization is extremely efficient, highly modular, unconditionally stable, and inherently robust.
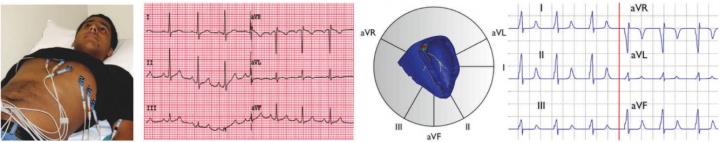
figure 1. validation of the new multi-scale simulation tool in terms of EKGs. in vivo acquired EKG recording based on a six-lead EKG (left), and in silico predicted EKG pattern based on a patient-specific whole heart model (right).
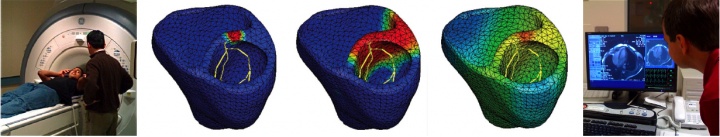
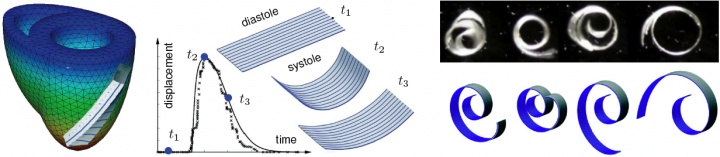
to calibrate and validate our model against clinical data, one of our students volunteered to have his EKG recording taken, see figure 1. to generate patient-specific anatomic models of the heart, we collaborate with professor michael mc connell in the the department of cardiovascular medicine. he took a series of MRI scans from which we generated a finite element mesh. figure 2 illustrates the time sequence of cardiac excitation simulated on the resulting patient-specific geometry. For the first time, we were able to generate an {\it{in silico}} EKG from the integration of all flux vectors projected onto six characteristic directions in space, see Fig.\,\ref{figure2}. Our collaborators in Cardiology are excited about the incredible agreement between the {\it{in vivo}} recorded EKG, left, and the {\it{in silico}} predicted EKG, right. They plan to use our algorithms for both student education and new therapy design.
Patient-specific simulation of cardiac electrophysiology. MRI scan of human heart (left), simulated time sequence of cardiac excitation (middle), and diagnostic MRI images to generate anatomic human heart model (right).