research statement
one of the most challenging applications of the mechanics of solid materials today is certainly the field of computational biomechanics, a well-recognized, fast-growing but not yet clearly defined subject that is unquestionably an interdisciplinary science par excellence. It provides a vast number of new and fascinating areas of application such as the internal and external remodeling of bones, the healing of fracture, the growth of tumors, wound healing of the epidermis, the regeneration of microdamaged muscles, functional adaptation and general repair processes of the cardiovascular system to name but a few. besides a basic knowledge in medicine, biology and chemistry, research in the field of computational biomechanics requires a profound theoretical background in thermodynamics, continuum mechanics and structural mechanics paired with the ability to develop efficient robust and stable computational simulation tools.
my ultimate goal is to establish an interactive computer-based biomechanical lab that supports the solution of medically and technologically challenging biomechanical problems.
selected research accomplishments
in contrast to traditional engineering materials, living organisms show the remarkable ability to adapt not only their geometry, but also their internal architecture and their material properties to environmental changes. although this functional adaptation of biological tissues has been known since julius wolff published his fundamental law of bone remodeling in 1892, research in biomechanics mainly focuses on the passive behavior of tissues rather than taking into account their active response to changes in mechanical loading. instead of following mainstream research and applying standard classical constitutive equations to biological materials, my personal research was driven by the desire to formulate appropriate continuum equations that properly account for the functional adaptation of living tissues. this adaptation is known to occur in three different forms which have dominated my previous research:
| density growth |
|---|
| when characterizing engineering structures with the help of classical continuum field theories,
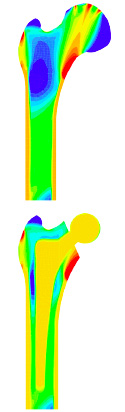
we typically think of closed thermodynamic systems for which the amount of matter within a fixed material domain is constant throughout the entire thermodynamical process. the appropriate characterization of living biological structures, however, goes far beyond this traditional point of view. it falls within the framework of open system thermodynamics. in order to account for biological growth, the traditional balance of mass is enriched by additional mass source and flux terms that account for cell growth, cell shrinkage, cell division or cell migration on a phenomenological level. it is obvious, that the exchange of matter with the environment not only affects the balance of mass itself since the newly generated or inflowing mass typically carries a specific amount of momentum, energy and entropy. as part of my habilitation research, I have developed a general theoretical framework for open system thermodynamics which has been implemented in a finite element based simulation tool for density growth in biological tissues. the results of this research have been documented a number of journal publications and in the habilitation thesis itself. for example, a medically relevant application of this model that has led to a pronounced industrial interest in our work is the optimization of modern implant design in hip replacement surgery. |
| Link 1 |
| Link 2 |
%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% $\circ$ \; {\sc{Volume Growth}}\\[2.pt] %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% While growth phenomena in hard biological tissue can essentially be attributed to local changes in density, growth in soft biological tissues typically takes place in the form of huge volumetric changes. Motivated by the classical ideas in large strain plasticity, we have adopted the {\hg{concept of a fictitious configuration}} to characterize the kinematics of growth. %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% \begin{figure}[h] \setlength{\unitlength}{1mm} \begin{picture}(0,30) \put( 4.0, 3.0){\includegraphics*[width=5.0cm,angle=0]{PICTURES/aorta3D200}} \put( 54.0, 3.0){\includegraphics*[width=5.0cm,angle=0]{PICTURES/aorta3D800}} \put(104.0, 3.0){\includegraphics*[width=5.0cm,angle=0]{PICTURES/aorta3D1600}} \put( 0.0, -2.0){\sffamily\bfseries\footnotesize{Figure 1 \bl Patient specific simulation of atherosclerotic plaque growth}} \end{picture} \end{figure} %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% Together with one of my PhD students, we have developed an efficient simulation tool for volumetric growth which has been applied to the simulation of {\hg{atherosclerosic plaque growth}}. Only recently, one of our master students implemented the model in a commercial finite element package and applied it to the simulation of {\hg{in-stent restenosis}}. The results of the simulation which are based on {\hg{patient-specific geometries}} generated from computer tomography have recently been published in two journal publications, see Figure 1.\\[8.pt] %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% $\circ$ \; {\sc{Microstructural Remodeling}}\\[2.pt] %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% By virtue of their collagenous microstructure, soft biological tissues show a pronounced anisotropic response. In the early stages of tissue development, the underlying collagenous scaffold is found to reorient itself with respect to the principal loading directions. In a corresponding continuum model, characteristic microstructural orientations can be represented by fiber vectors. Guided by minimization principles, we have developed a concept of {\hg{fiber reorientation}} in which a single characteristic microstructural orientation is allowed to gradually align with the maximum principal strain direction. %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% \begin{figure}[h] \unitlength1.0mm \begin{picture}(150.0,27.0) \put(42.0,30.0){\includegraphics[height=72.0mm,angle=270]{PICTURES/tendon_title}} \put( 0.0,0.0){\sffamily\bfseries\footnotesize{Figure 2 \bl Simulation of fiber remodeling in ex-vivo grown tendon in terms of reorienation of collagenous chain network}} \end{picture} \end{figure} %%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%%% A typical application of this model can be found within the field of {\hg{tissue engineering}} where tissue replacements are grown outside the human body. Due to the absence of mechanical loading, these in vitro engineered functional tissue constructs typically lack a pronounced microstructural orientation. However, the formation of this orientation can be stimulated by subjecting the growing tissue to different mechanical loading scenarios. In close collaboration with experts in tissue engineering from the University of Michigan, we have been able to qualitatively validate our fiber reorientation model, see Figure 2. The long term goal of this project is the {\hg{optimal stimulation of microstructural growth}}. Conceptually speaking, we strive for finding the optimal amount, frequency and direction of loading with the aim of reproducing the in vivo conditions as realistic as possible.
current projects
| sunt in culpa |
|---|
| "Lorem ipsum dolor sit amet, consectetur adipisicing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur. Excepteur sint occaecat cupidatat non proident, sunt in culpa qui officia deserunt mollit anim id est laborum." |
| Link 1 |
| Link 2 |