cardiovascular disease is the primary cause of death in the industrialized world, generating annual health care costs in excess of $430 billion in the united states alone. in spite of tremendous scientific improvements during the past 20 years, heart failure remains one of the most common, costly, disabling, and deadly medical conditions affecting more than 25 million people world wide. repairing the diseased heart is a desirable but elusive goal. traditionally, the development of cardiac therapies has been experience-based empirical rather than simulation-based predictive. advanced continuum theories combined with modern imaging modalities and computational techniques now offer the potential to provide greater insight into the complex pathways of cardiac disease, and thereby guide the design of new successful treatment strategies.
our goal is to establish computer-guided surgical planning in cardiac disease using novel multi-scale continuum theories of excitation, contraction, and remodeling.
in the united states alone, almost half a million people die each year as a result of cardiac arrhythmias.
in the healthy heart, rhythmic cardiac contraction is coordinated
through smoothly propagating electrical waves of excitation.
to model the spatio-temporal evolution of cardiac excitation, we adopt the modified fitzhugh-nagumo equations for excitable cells.
they define a system of coupled evolution equations for the fast action potential
and the slow recovery variable which phenomenologically summarizes the effects of all ionic currents across the cell membrane.
we have developed a novel finite-element based solution technique in which the action potential is introduced globally as a nodal degree of freedom, whereas the recovery variable is treated locally as an internal variable on the integration point level
[49]. paired with an implicit time integration scheme and an incremental iterative newton-raphson solution strategy, this particular discretization is extremely efficient, highly modular, unconditionally stable, and inherently robust [55].
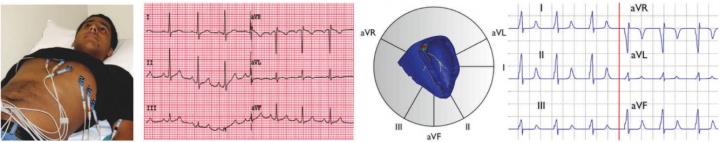
figure 1. validation of the new multi-scale simulation tool in terms of EKGs. in vivo acquired EKG recording based on a six-lead EKG (left), and in silico predicted EKG pattern based on a patient-specific whole heart model (right).
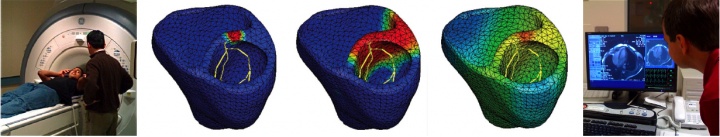
to calibrate and validate our model against clinical data, one of our students volunteered to have his EKG recording taken, see figure 1. to generate patient-specific anatomic models of the heart, we collaborate with professor michael mc connell in cardiovascular medicine. he took a series of MRI scans from which we generated a finite element mesh. figure 2 illustrates the time sequence of cardiac excitation simulated on the resulting patient-specific geometry. for the first time, we were able to generate an in silico EKG from the integration of all flux vectors projected onto six characteristic directions in space, see figure 1 [51]. we are excited about the incredible agreement between the in vivo recorded EKG, left, and the in silico predicted EKG, right. together with our collaborators in cardiac medicine, we plan to use our algorithms for both student education and new therapy design.
figure 2. patient-specific simulation of cardiac electrophysiology. MRI scan of human heart (left), simulated time sequence of cardiac excitation (middle), and diagnostic MRI images to generate anatomic human heart model (right).
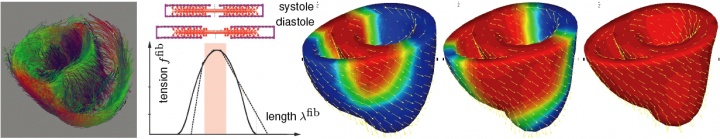
electric excitation induces an increased calcium concentration within the cell, which, in turn, initiates cellular contraction through actin-myosin filament sliding. to simulate these effects, existing simulation tools combine a finite-difference based excitation model with a finite-element based contraction model, having to pass information back and forth between both models at every instant in time. to reduce the risk of inherent instabilities associated with this explicit approach, existing simulation tools require an extremely fine spatial and temporal discretization. taking advantage of our core competence in multi-physics modeling, we have developed a fully coupled multi-field finite element approach to monolithically solve the electrical excitation problem and the mechanical equilibrium problem. this model can directly incorporate experimentally measured tension-length relations by mapping them onto patient-specific fiber orientations determined non-invasively through diffusion tensor MRI, see figure 3. for the first time, we have demonstrated a unified, fully coupled, extremely efficient, and robust solution technique that can reliably predict excitation-contraction patterns on single desktop computers with simulation times on the order of minutes.
figure 3. fiber orientation map from diffusion tensor MRI adopted from zhukov & barr [2003] (left), actin-myosin based filament sliding model with characteristic tension-length relation (middle), and simulation of excitation-contraction coupling in terms of bi-ventricular heart model with generic fiber orientation (right).
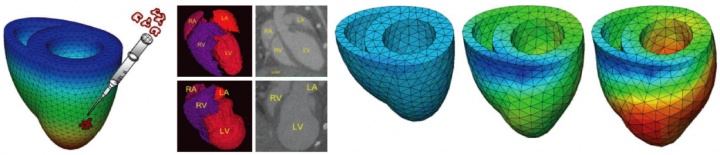
a leading cause of heart failure is myocardial infarction caused by the local death of heart muscle cells due to insufficient blood supply. as a result, the functional units of the myocardium, the cardiomyocytes, lose their contractile property and die. recently, stem cell therapy has emerged as a promising methodology for cardiac repair by injecting cells into the damaged tissue. in close collaboration with professors chris zarins in vascular surgery and sarah heilshorn in material sciences, we have developed computational tools to simulate the progression of infarct-induced heart failure to predict optimal cell injection sites. the underlying continuum model is based on the multiplicative decomposition of the deformation gradient into an elastic part and a growth part [25,54]. in the past, we have successfully applied this model in the context of open system thermodynamics [12,13,14,15] to simulate progressive plaque growth in arteries [34]. together with professor andreas menzel from the tu dortmund, germany [41], professor gerhard holzapfel from the tu graz, austria, and professors krishna garikipati and ellen arruda from the university of michigan, we have also developed algorithms to predict remodeling in arteries [36] and tendons [27,29]. we have now developed a new macroscopic growth and remodeling law for cardiac tissue motivated by microscopic changes in cardiomyocyte geometry that can be quantified experimentally through tissue histology. figure 4 shows our simulation of infarct-induced growth motivated by micro-ct images of mouse infarct models provided by our collaborator joe wu in radiology.
figure 4. virtual injection of stem-cell derived cardiomyocytes into the damaged myocardium (left). micro-ct images of infarct-induced growth in mouse model adopted from doyle et al. [2007] (middle). finite element simulation of infarct-induced growth (right).
recent studies in the wu lab have shown that stem cells may offer regenerative potential in infarct-induced heart failure. however, randomly injected cells lack the organization and physical structure required to create a cohesive, uniform tissue with continuous, synchronized beating. through a multidisciplinary collaboration with professors joe wu in radiology, chris zarins in vascular surgery, sarah heilshorn in material sciences, and beth pruitt in mechanical engineering, we are investigating the potential to engineer actively contracting stem-cell based tissue grafts to re-establish cardiac contraction and prevent heart failure. figure 5 displays our multi-scale finite element model of actively contracting cardiomyocytes seeded on a polymeric base layer derived in a collaboration with professor markus boel from the tu braunschweig, germany [48]. the in silico designed tissue constructs were calibrated, validated, and verified against in vitro engineered muscular thin films cultured by our collaborator professor kit parker at harvard university. our model is used to optimize the functional integration of a multi-layered tissue patch upon its implantation onto the ventricular wall.
figure 5. virtual implantation of tissue engineered vascular grafts onto the damaged myocardium (left). actively contracting cardiomyocytes seeded on polymeric base layer, experiment (top) vs. finite element simulation (bottom).
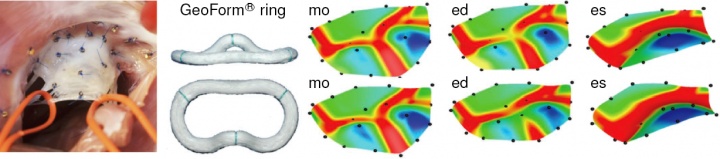
mitral regurgitation is a progressive, valvular disorder affecting 4 million people in the united states alone. the most common surgical approach to repair a leaking valve is to bring the leaflets closer together using annuloplasty rings. annuloplasty rings come in different shapes, materials, and sizes. choosing the optimal ring type largely depends on the surgeon's experience and personal preference. it is hypothesized that annuloplasty rings influence leaflet curvature, which in turn may considerably impact repair durability. in an attempt to guide the optimal ring choice and improve repair devices, we have quantified leaflet curvature in beating ovine hearts using in vivo acquired videofluoroscopic marker data provided by our collaborator professor craig miller in cardiothoracic surgery [56]. figure 6 documents the acute impact of annuloplasty ring implantation on the maximum principal leaflet curvature determined with our custom-made subdivision surface algorithm. using the same data set in an inverse finite element analysis has allowed us, for the first time, to identify the material parameters of the mitral valve in the beating heart [46]. a co-advised student in the lab of professor craig miller and professor neil ingels from the palo alto medical foundation identified the in vivo stiffness to be an order of magnitude larger than the in vitro measured tissue stiffness [50,52].
figure 6. intraoperative photograph showing 23 tantalium markers sewn on the mitral valve leaflet (left), annuloplasty ring (middle), and maximum principal curvature distribution in the leaflet with ring (top) and without ring (bottom).
our vision is to revolutionize thinking in regenerative medicine and induce a paradigm shift from empirical to predictive therapy design using multi-scale, multi-physics models derived from first principles.